
Question Number 69159 by Rio Michael last updated on 20/Sep/19
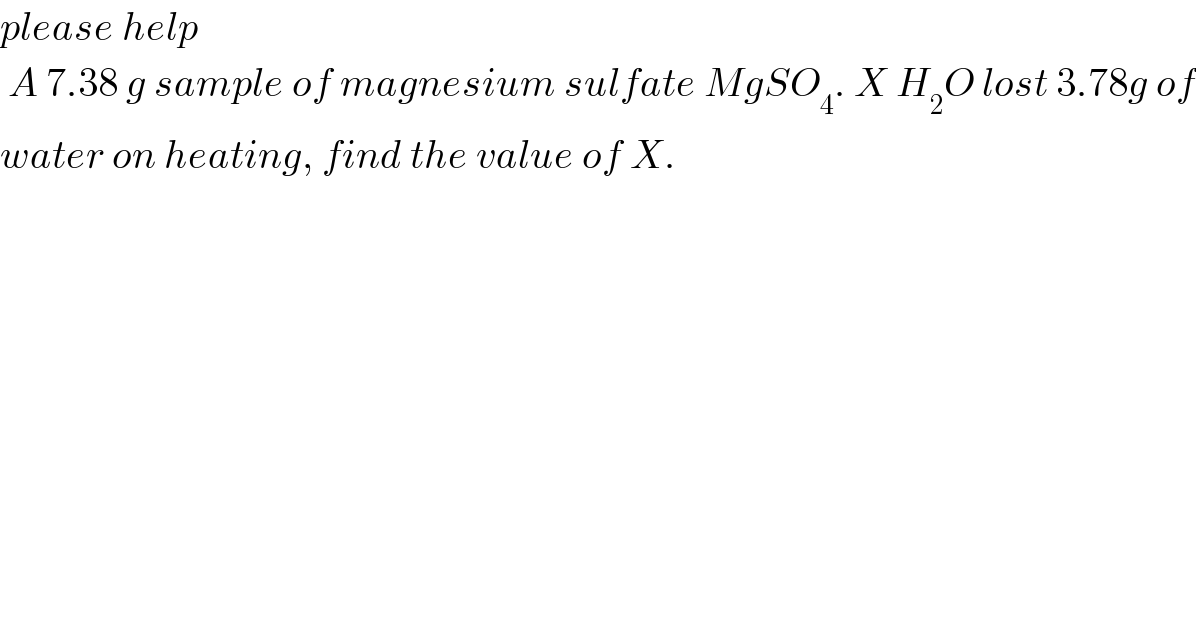
$${please}\:{help}\: \\ $$$$\:{A}\:\mathrm{7}.\mathrm{38}\:{g}\:{sample}\:{of}\:{magnesium}\:{sulfate}\:{MgSO}_{\mathrm{4}} .\:{X}\:{H}_{\mathrm{2}} {O}\:{lost}\:\mathrm{3}.\mathrm{78}{g}\:{of} \\ $$$${water}\:{on}\:{heating},\:{find}\:{the}\:{value}\:{of}\:{X}. \\ $$
