
States of MatterQuestion and Answers: Page 1
Question Number 220583 Answers: 0 Comments: 0

Question Number 212018 Answers: 0 Comments: 0

Question Number 203879 Answers: 1 Comments: 0
Question Number 203878 Answers: 1 Comments: 0
Question Number 180902 Answers: 1 Comments: 0
Question Number 133438 Answers: 0 Comments: 0

Question Number 116238 Answers: 1 Comments: 0
Question Number 92621 Answers: 0 Comments: 1

Question Number 80164 Answers: 0 Comments: 0

Question Number 77891 Answers: 0 Comments: 0
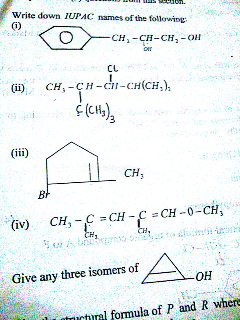
Question Number 44089 Answers: 0 Comments: 0

Question Number 42663 Answers: 0 Comments: 0
Question Number 38478 Answers: 0 Comments: 1
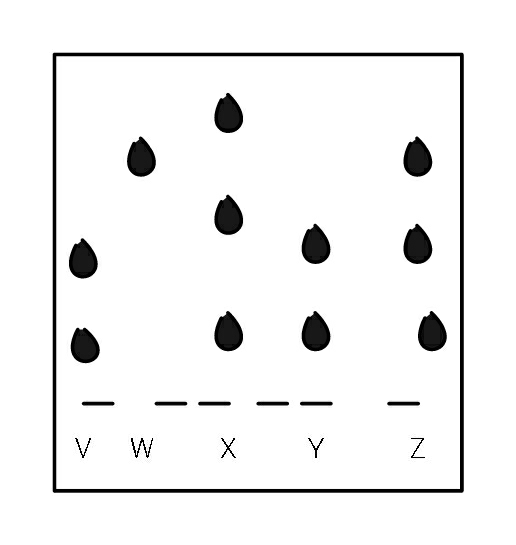
Question Number 35197 Answers: 1 Comments: 0

