
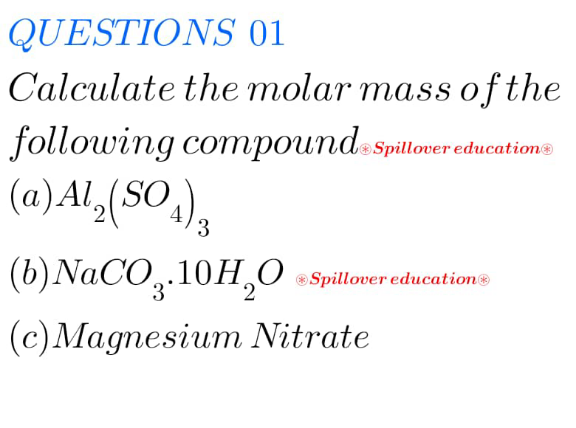
Question Number 217593 by Spillover last updated on 16/Mar/25
Answered by mahdipoor last updated on 16/Mar/25
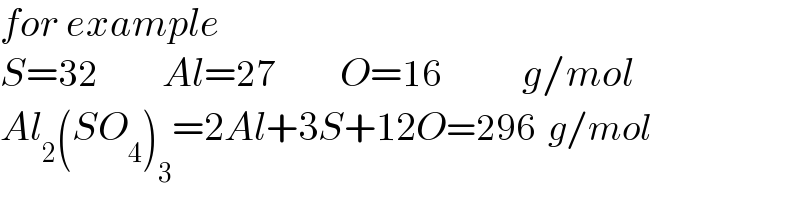
$${for}\:{example}\: \\ $$$${S}=\mathrm{32}\:\:\:\:\:\:\:\:{Al}=\mathrm{27}\:\:\:\:\:\:\:\:{O}=\mathrm{16}\:\:\:\:\:\:\:\:\:\:{g}/{mol} \\ $$$${Al}_{\mathrm{2}} \left({SO}_{\mathrm{4}} \right)_{\mathrm{3}} =\mathrm{2}{Al}+\mathrm{3}{S}+\mathrm{12}{O}=\mathrm{296}\:\:{g}/{mol} \\ $$
Commented by Spillover last updated on 16/Mar/25

$${thanks} \\ $$
Answered by Spillover last updated on 16/Mar/25
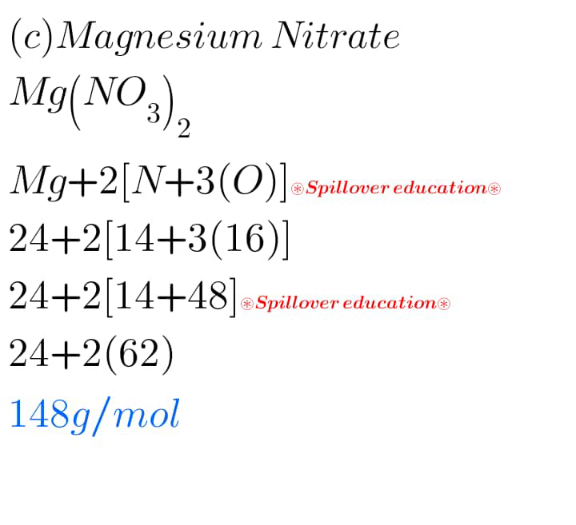
Answered by Spillover last updated on 16/Mar/25
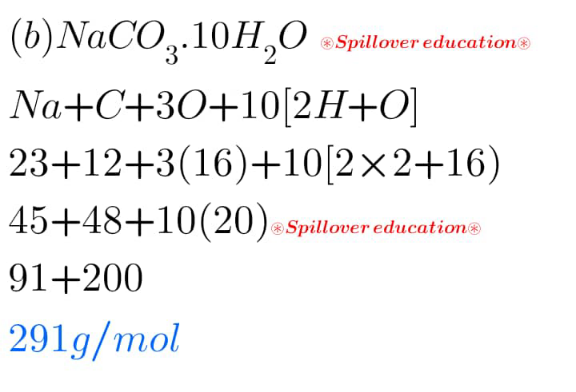
Answered by Spillover last updated on 16/Mar/25

