
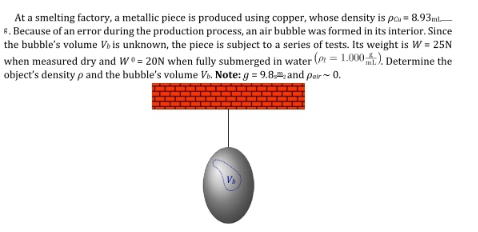
Question Number 195721 by Humble last updated on 08/Aug/23
Answered by mr W last updated on 08/Aug/23
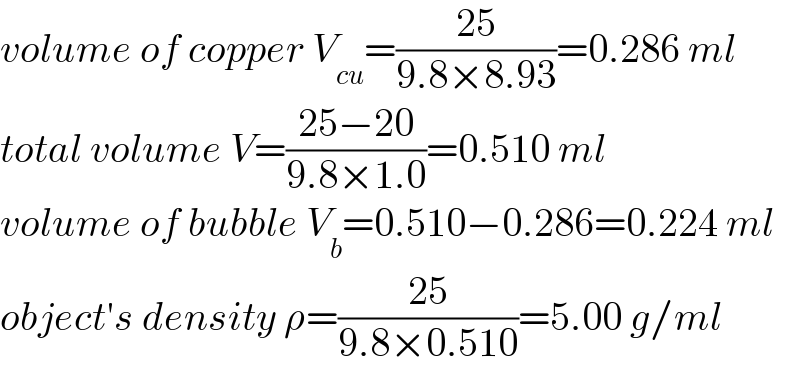
$${volume}\:{of}\:{copper}\:{V}_{{cu}} =\frac{\mathrm{25}}{\mathrm{9}.\mathrm{8}×\mathrm{8}.\mathrm{93}}=\mathrm{0}.\mathrm{286}\:{ml} \\ $$$${total}\:{volume}\:{V}=\frac{\mathrm{25}−\mathrm{20}}{\mathrm{9}.\mathrm{8}×\mathrm{1}.\mathrm{0}}=\mathrm{0}.\mathrm{510}\:{ml} \\ $$$${volume}\:{of}\:{bubble}\:{V}_{{b}} =\mathrm{0}.\mathrm{510}−\mathrm{0}.\mathrm{286}=\mathrm{0}.\mathrm{224}\:{ml} \\ $$$${object}'{s}\:{density}\:\rho=\frac{\mathrm{25}}{\mathrm{9}.\mathrm{8}×\mathrm{0}.\mathrm{510}}=\mathrm{5}.\mathrm{00}\:{g}/{ml} \\ $$
Commented by Humble last updated on 09/Aug/23

$$\mathrm{Great}!! \\ $$
