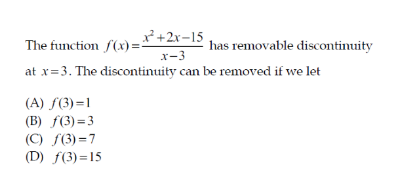OthersQuestion and Answers: Page 38
Question Number 154022 Answers: 0 Comments: 0
Question Number 153685 Answers: 3 Comments: 2

Question Number 153438 Answers: 0 Comments: 2

Question Number 153464 Answers: 3 Comments: 1

Question Number 153345 Answers: 1 Comments: 0

Question Number 153312 Answers: 0 Comments: 3

Question Number 153006 Answers: 1 Comments: 8
$${prove}\:{that}\: \\ $$$$\mathrm{4}!!=\mathrm{8} \\ $$$${please}\:{help} \\ $$
Question Number 152899 Answers: 0 Comments: 0

Question Number 152857 Answers: 0 Comments: 0
Question Number 152845 Answers: 0 Comments: 0

Question Number 152826 Answers: 1 Comments: 0
Question Number 152748 Answers: 1 Comments: 0

Question Number 152723 Answers: 1 Comments: 0
Question Number 152672 Answers: 1 Comments: 0
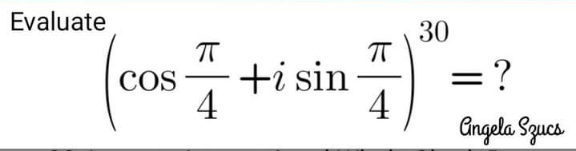
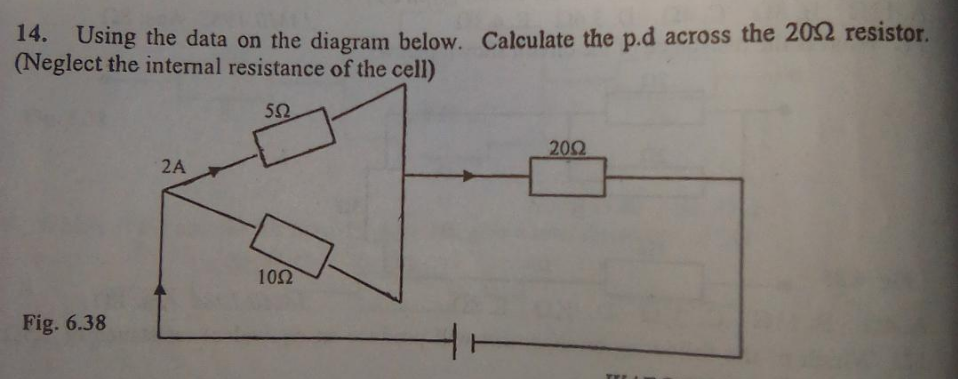
Question Number 152532 Answers: 1 Comments: 0

Question Number 152450 Answers: 1 Comments: 1

Question Number 152447 Answers: 1 Comments: 1

Question Number 152445 Answers: 0 Comments: 1

Question Number 152443 Answers: 0 Comments: 2
Question Number 152441 Answers: 3 Comments: 1

Question Number 152439 Answers: 0 Comments: 3

Question Number 152437 Answers: 1 Comments: 1
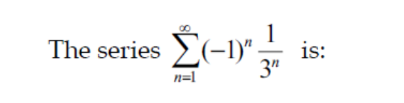
Question Number 152435 Answers: 1 Comments: 1
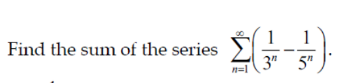
Question Number 152433 Answers: 1 Comments: 1

Question Number 152431 Answers: 0 Comments: 3

Question Number 152429 Answers: 1 Comments: 1