
OthersQuestion and Answers: Page 131
Question Number 23823 Answers: 1 Comments: 0
Question Number 23773 Answers: 0 Comments: 2
Question Number 23715 Answers: 0 Comments: 0
Question Number 23711 Answers: 1 Comments: 0
Question Number 23707 Answers: 1 Comments: 4
Question Number 24506 Answers: 0 Comments: 8
Question Number 23694 Answers: 0 Comments: 0
Question Number 23739 Answers: 0 Comments: 3

Question Number 23666 Answers: 2 Comments: 1
Question Number 23645 Answers: 0 Comments: 1
Question Number 23632 Answers: 0 Comments: 9

Question Number 23617 Answers: 2 Comments: 3
Question Number 23559 Answers: 1 Comments: 1
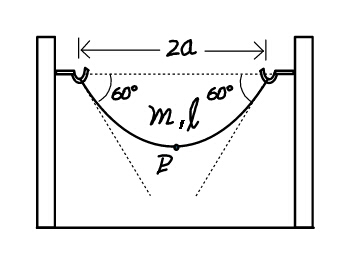
Question Number 23556 Answers: 1 Comments: 0
Question Number 23548 Answers: 0 Comments: 3

Question Number 23585 Answers: 0 Comments: 0
Question Number 23531 Answers: 1 Comments: 3

Question Number 23520 Answers: 0 Comments: 3
Question Number 23492 Answers: 0 Comments: 5
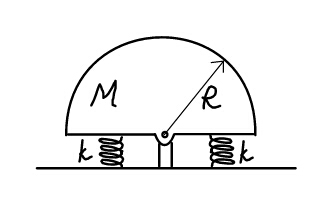
Question Number 23489 Answers: 1 Comments: 2
Question Number 23488 Answers: 0 Comments: 0
$${area}\:{of}\:{a}\left(\mathrm{1}−\mathrm{cos}\:\theta\right) \\ $$
Question Number 23481 Answers: 0 Comments: 5
Question Number 23476 Answers: 0 Comments: 0
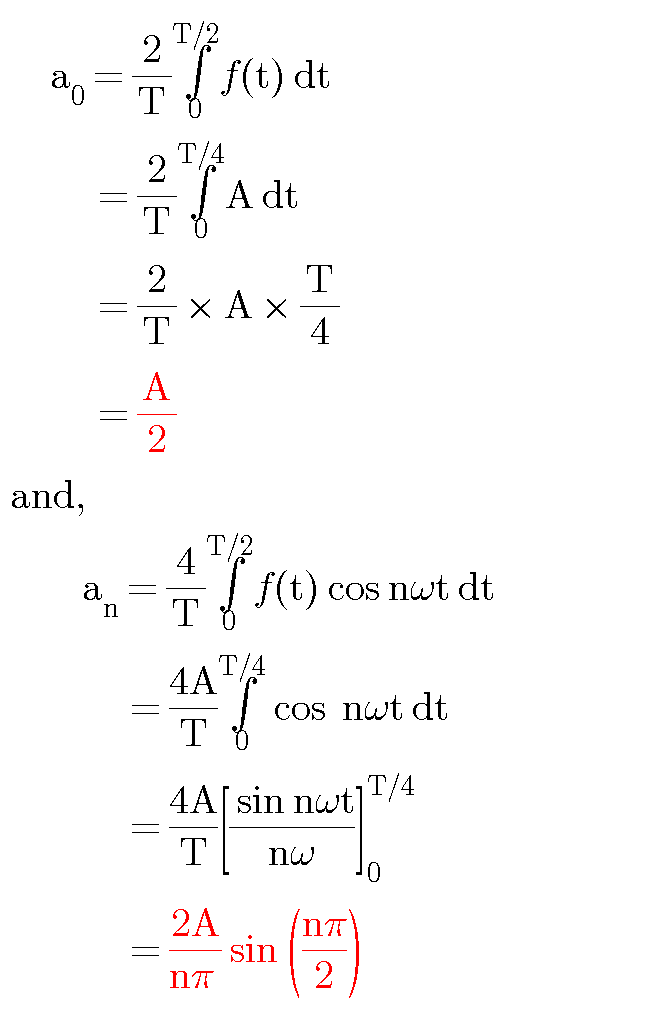
Question Number 23472 Answers: 1 Comments: 1
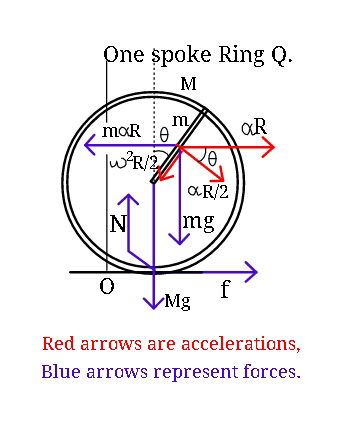
Question Number 23458 Answers: 0 Comments: 1

Question Number 23444 Answers: 0 Comments: 0

Pg 126 Pg 127 Pg 128 Pg 129 Pg 130 Pg 131 Pg 132 Pg 133 Pg 134 Pg 135
