
Previous in Heat and Theromdynamics Next in Heat and Theromdynamics
Question Number 42262 by Necxx last updated on 21/Aug/18

$${Heat}\:{is}\:{supplied}\:{at}\:{a}\:{rate}\:{of}\:\mathrm{500}{W} \\ $$$${to}\:{a}\:{pressure}\:{cooker}\:{containing} \\ $$$${water}\:{and}\:{fitted}\:{with}\:{a}\:{safety}\: \\ $$$${valve}.{Steam}\:{escape}\:{such}\:{that}\: \\ $$$${water}\:{is}\:{lost}\:{at}\:\mathrm{12}{g}/{min}.{If}\:{the} \\ $$$${heat}\:{is}\:{supplied}\:{at}\:\mathrm{900}{W},{water}\:{is} \\ $$$${lost}\:{at}\:\mathrm{20}{g}/{min}.{Calculate}\:{the} \\ $$$${specific}\:{latent}\:{heat}\:{of}\:{steam}. \\ $$$$ \\ $$
Answered by tanmay.chaudhury50@gmail.com last updated on 21/Aug/18
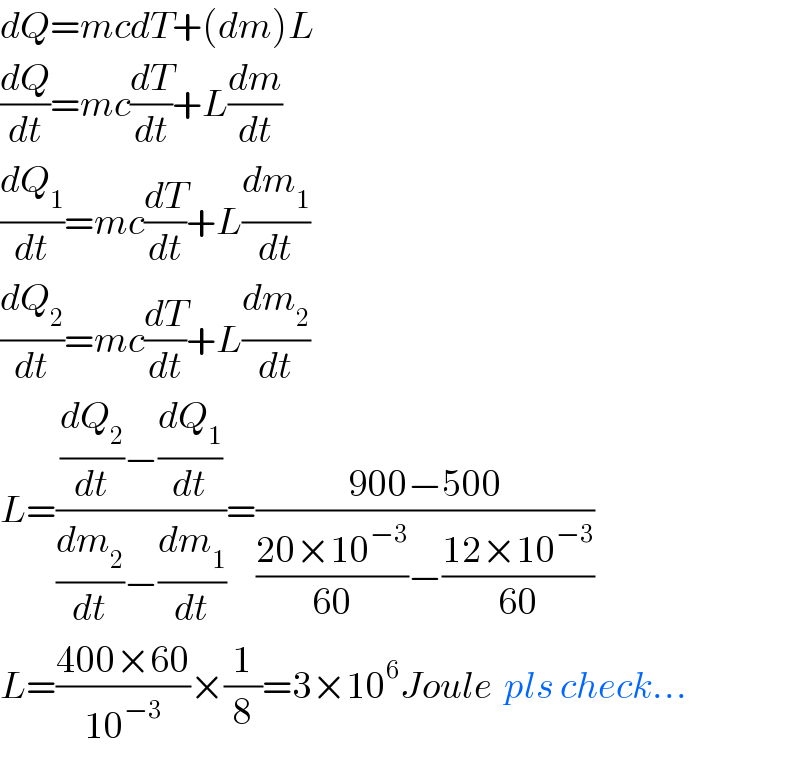
$${dQ}={mcdT}+\left({dm}\right){L} \\ $$$$\frac{{dQ}}{{dt}}={mc}\frac{{dT}}{{dt}}+{L}\frac{{dm}}{{dt}} \\ $$$$\frac{{dQ}_{\mathrm{1}} }{{dt}}={mc}\frac{{dT}}{{dt}}+{L}\frac{{dm}_{\mathrm{1}} }{{dt}} \\ $$$$\frac{{dQ}_{\mathrm{2}} }{{dt}}={mc}\frac{{dT}}{{dt}}+{L}\frac{{dm}_{\mathrm{2}} }{{dt}} \\ $$$${L}=\frac{\frac{{dQ}_{\mathrm{2}} }{{dt}}−\frac{{dQ}_{\mathrm{1}} }{{dt}}}{\frac{{dm}_{\mathrm{2}} }{{dt}}−\frac{{dm}_{\mathrm{1}} }{{dt}}}=\frac{\mathrm{900}−\mathrm{500}}{\frac{\mathrm{20}×\mathrm{10}^{−\mathrm{3}} }{\mathrm{60}}−\frac{\mathrm{12}×\mathrm{10}^{−\mathrm{3}} }{\mathrm{60}}} \\ $$$${L}=\frac{\mathrm{400}×\mathrm{60}}{\mathrm{10}^{−\mathrm{3}} }×\frac{\mathrm{1}}{\mathrm{8}}=\mathrm{3}×\mathrm{10}^{\mathrm{6}} {Joule}\:\:{pls}\:{check}... \\ $$
Commented by Necxx last updated on 21/Aug/18
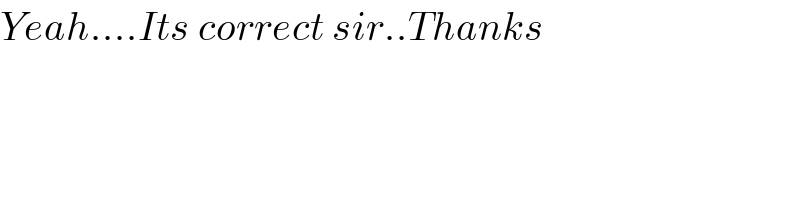
$${Yeah}....{Its}\:{correct}\:{sir}..{Thanks} \\ $$
