
Heat and TheromdynamicsQuestion and Answers: Page 1
Question Number 214780 Answers: 0 Comments: 3
Question Number 206171 Answers: 0 Comments: 0

Question Number 206168 Answers: 0 Comments: 1

Question Number 200129 Answers: 0 Comments: 0

Question Number 194194 Answers: 0 Comments: 0

Question Number 186028 Answers: 0 Comments: 2
$$ \\ $$A wolf weighs 40 kg, how many kilo calories does it need to maintain its body temperature?
Question Number 178587 Answers: 0 Comments: 0

Question Number 175652 Answers: 0 Comments: 0

Question Number 166067 Answers: 0 Comments: 2

Question Number 164137 Answers: 0 Comments: 1
$${what}\:{is}\:{the}\:{triple}\:{point}\:{of}\:{water}? \\ $$
Question Number 141111 Answers: 1 Comments: 1
Question Number 137317 Answers: 0 Comments: 3
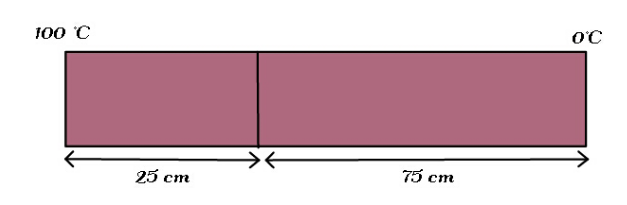
Question Number 136487 Answers: 0 Comments: 1
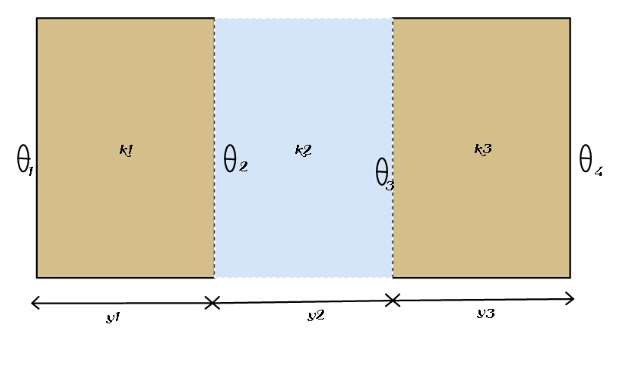
Question Number 131672 Answers: 0 Comments: 0
$$\Delta{t}=\mathrm{210}{C}^{°} \:\:\:\:\:\:\:\:\:{k}=? \\ $$
Question Number 127423 Answers: 0 Comments: 0

Question Number 127336 Answers: 0 Comments: 0

Question Number 124414 Answers: 0 Comments: 0
Question Number 119452 Answers: 1 Comments: 1

Question Number 111388 Answers: 0 Comments: 0

Question Number 110550 Answers: 0 Comments: 0

Question Number 103484 Answers: 0 Comments: 0

Question Number 103409 Answers: 0 Comments: 1
Question Number 94922 Answers: 1 Comments: 0
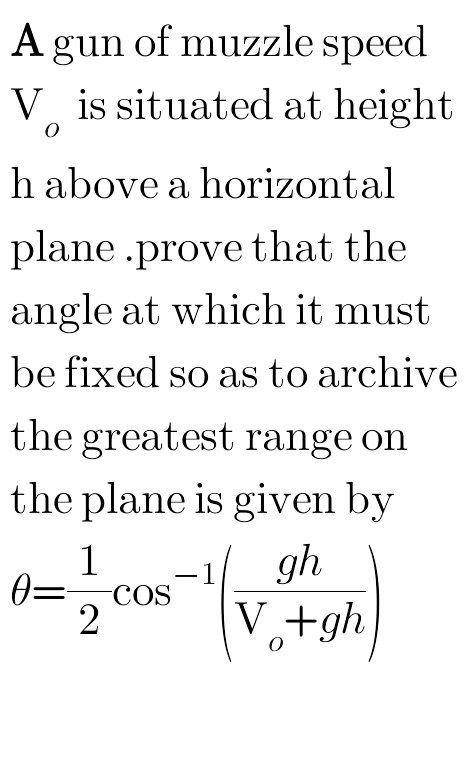
Question Number 90073 Answers: 0 Comments: 0
Question Number 75648 Answers: 0 Comments: 0
Question Number 75204 Answers: 0 Comments: 0

