
Question Number 203879 by Spillover last updated on 31/Jan/24
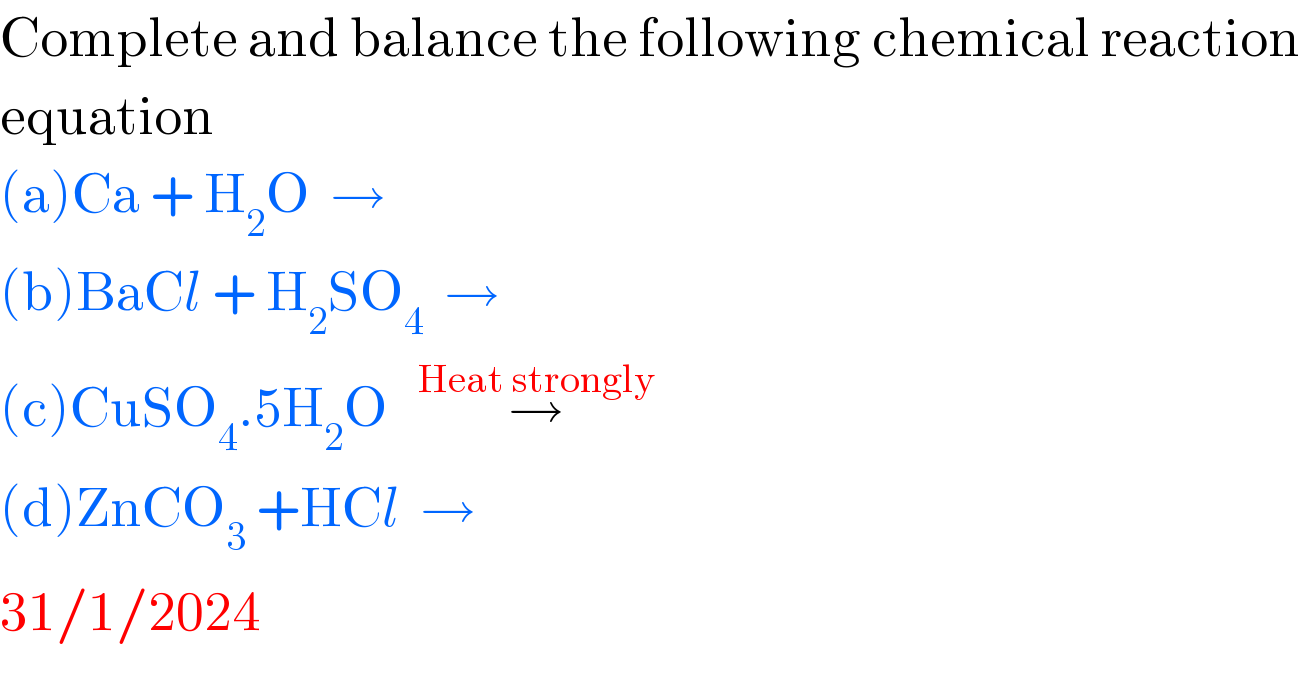
$$\mathrm{Complete}\:\mathrm{and}\:\mathrm{balance}\:\mathrm{the}\:\mathrm{following}\:\mathrm{chemical}\:\mathrm{reaction}\: \\ $$$$\mathrm{equation} \\ $$$$\left(\mathrm{a}\right)\mathrm{Ca}\:+\:\mathrm{H}_{\mathrm{2}} \mathrm{O}\:\:\rightarrow\:\: \\ $$$$\left(\mathrm{b}\right)\mathrm{BaC}{l}\:+\:\mathrm{H}_{\mathrm{2}} \mathrm{SO}_{\mathrm{4}} \:\:\rightarrow \\ $$$$\left(\mathrm{c}\right)\mathrm{CuSO}_{\mathrm{4}} .\mathrm{5H}_{\mathrm{2}} \mathrm{O}\:\:\:\overset{\mathrm{Heat}\:\mathrm{strongly}} {\rightarrow} \\ $$$$\left(\mathrm{d}\right)\mathrm{ZnCO}_{\mathrm{3}} \:+\mathrm{HC}{l}\:\:\rightarrow \\ $$$$\mathrm{31}/\mathrm{1}/\mathrm{2024} \\ $$
Answered by Calculusboy last updated on 31/Jan/24
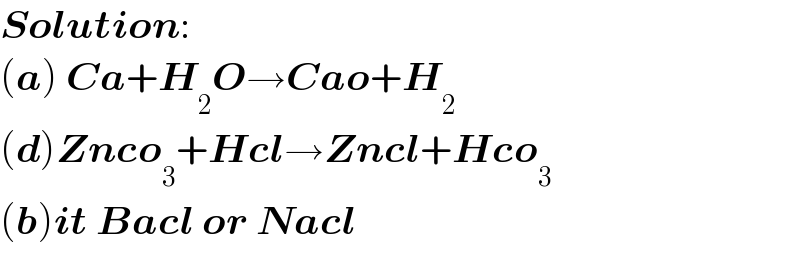
$$\boldsymbol{{Solution}}: \\ $$$$\left(\boldsymbol{{a}}\right)\:\boldsymbol{{Ca}}+\boldsymbol{{H}}_{\mathrm{2}} \boldsymbol{{O}}\rightarrow\boldsymbol{{Cao}}+\boldsymbol{{H}}_{\mathrm{2}} \\ $$$$\left(\boldsymbol{{d}}\right)\boldsymbol{{Znco}}_{\mathrm{3}} +\boldsymbol{{Hcl}}\rightarrow\boldsymbol{{Zncl}}+\boldsymbol{{Hco}}_{\mathrm{3}} \\ $$$$\left(\boldsymbol{{b}}\right)\boldsymbol{{it}}\:\boldsymbol{{Bacl}}\:\boldsymbol{{or}}\:\boldsymbol{{Nacl}} \\ $$
Commented by som(math1967) last updated on 01/Feb/24
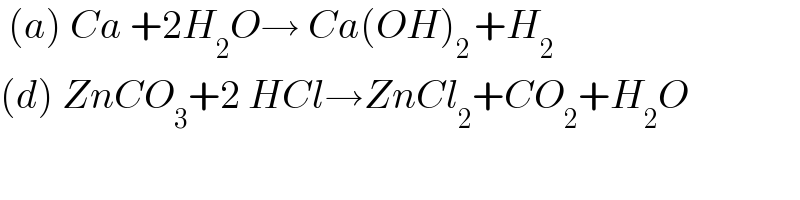
$$\:\left({a}\right)\:{Ca}\:+\mathrm{2}{H}_{\mathrm{2}} {O}\rightarrow\:{Ca}\left({OH}\right)_{\mathrm{2}\:} +{H}_{\mathrm{2}} \\ $$$$\left({d}\right)\:{ZnCO}_{\mathrm{3}} +\mathrm{2}\:{HCl}\rightarrow{ZnCl}_{\mathrm{2}} +{CO}_{\mathrm{2}} +{H}_{\mathrm{2}} {O} \\ $$
Commented by Spillover last updated on 11/Feb/24

$${correct} \\ $$
