
AllQuestion and Answers: Page 1848
Question Number 26224 Answers: 1 Comments: 1
Question Number 26218 Answers: 0 Comments: 2
$$\underset{\mathrm{2}} {\overset{\mathrm{4}} {\int}}\mathrm{sin}\theta\:{d}\theta \\ $$
Question Number 26207 Answers: 1 Comments: 0
Question Number 26205 Answers: 1 Comments: 1

Question Number 26200 Answers: 1 Comments: 0

Question Number 26198 Answers: 1 Comments: 0
Question Number 26192 Answers: 0 Comments: 3
Question Number 26191 Answers: 1 Comments: 0
$$\mathrm{2}\frac{{dy}}{{dx}}+{x}=\mathrm{4}\sqrt{{y}} \\ $$
Question Number 26181 Answers: 2 Comments: 0
Question Number 26179 Answers: 1 Comments: 0
Question Number 26176 Answers: 0 Comments: 1
Question Number 26174 Answers: 0 Comments: 0
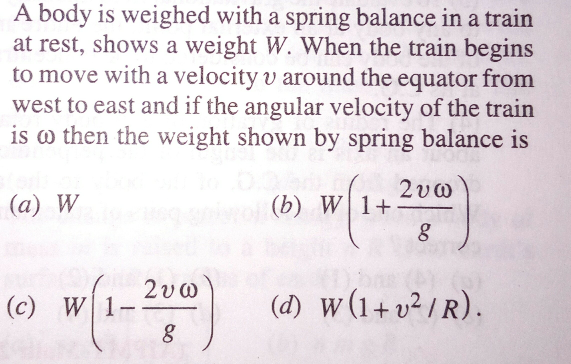
Question Number 26162 Answers: 1 Comments: 0
Question Number 26166 Answers: 0 Comments: 0

Question Number 26159 Answers: 1 Comments: 0
Question Number 26156 Answers: 1 Comments: 0
Question Number 26153 Answers: 1 Comments: 0
Question Number 26148 Answers: 2 Comments: 2

Question Number 26147 Answers: 1 Comments: 0
Question Number 26142 Answers: 0 Comments: 0
Question Number 26143 Answers: 2 Comments: 1
Question Number 26135 Answers: 1 Comments: 1
Question Number 26133 Answers: 1 Comments: 1
Question Number 26132 Answers: 0 Comments: 1
Question Number 26127 Answers: 1 Comments: 0
Question Number 26125 Answers: 0 Comments: 1
Pg 1843 Pg 1844 Pg 1845 Pg 1846 Pg 1847 Pg 1848 Pg 1849 Pg 1850 Pg 1851 Pg 1852
