
AllQuestion and Answers: Page 1515
Question Number 60313 Answers: 3 Comments: 1
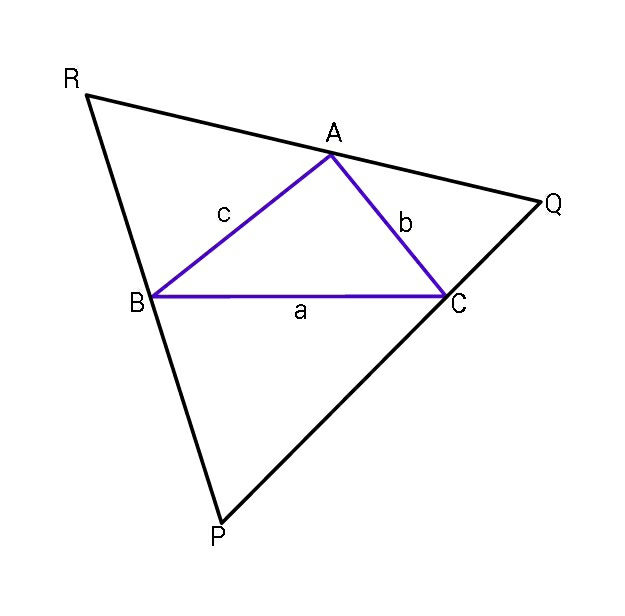
Question Number 60311 Answers: 1 Comments: 2
$$\int\frac{{dx}}{\sqrt{{sec}\:{h}^{\mathrm{2}} \left({x}\right)+\mathrm{1}}}\:{dx} \\ $$
Question Number 60308 Answers: 0 Comments: 0
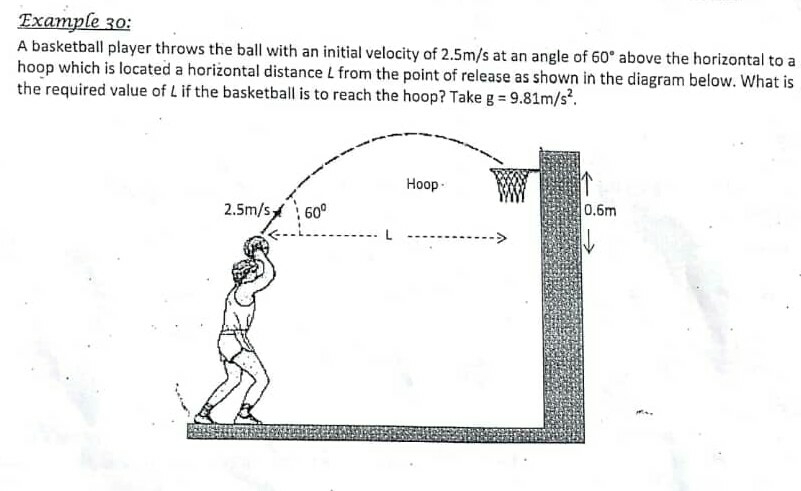
Question Number 60307 Answers: 0 Comments: 0
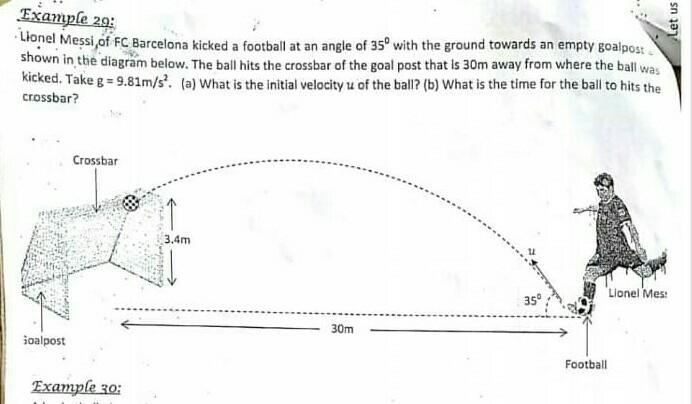
Question Number 60304 Answers: 0 Comments: 0
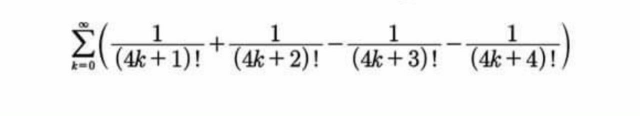
Question Number 60303 Answers: 1 Comments: 0

Question Number 60287 Answers: 2 Comments: 2
Question Number 60283 Answers: 1 Comments: 1
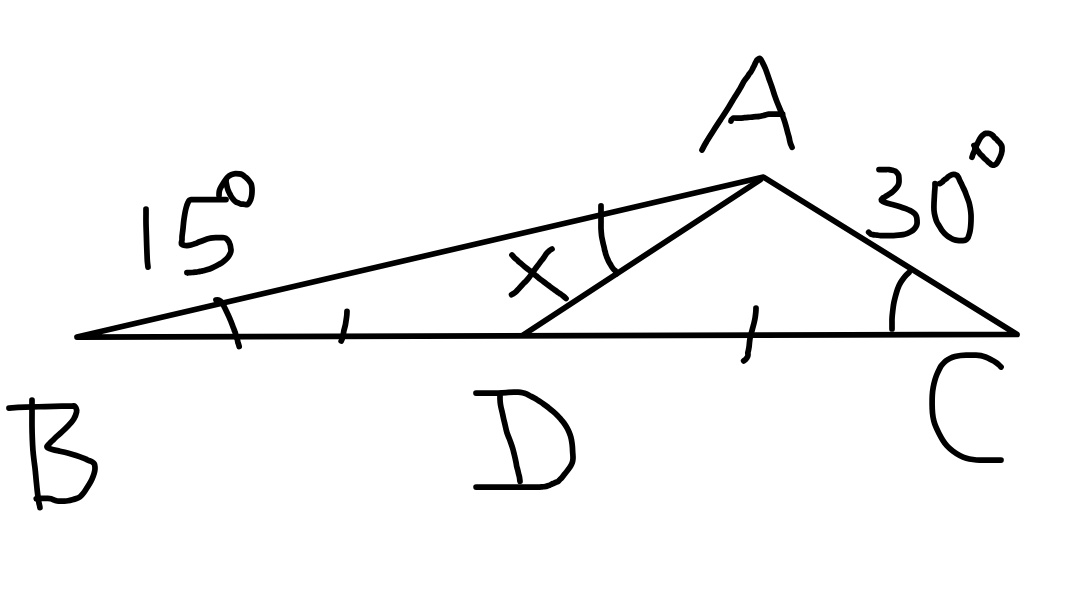
Question Number 60269 Answers: 3 Comments: 3
Question Number 60264 Answers: 0 Comments: 0
Question Number 60263 Answers: 0 Comments: 1
Question Number 60257 Answers: 0 Comments: 4
Question Number 60256 Answers: 1 Comments: 0

Question Number 60255 Answers: 0 Comments: 0
Question Number 60275 Answers: 0 Comments: 0

Question Number 60274 Answers: 0 Comments: 1

Question Number 60247 Answers: 0 Comments: 0
Question Number 60237 Answers: 0 Comments: 1

Question Number 60234 Answers: 1 Comments: 0
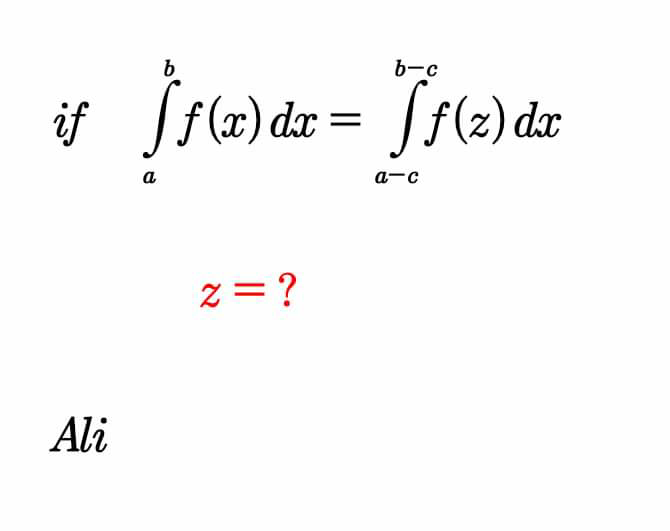
Question Number 60227 Answers: 0 Comments: 0
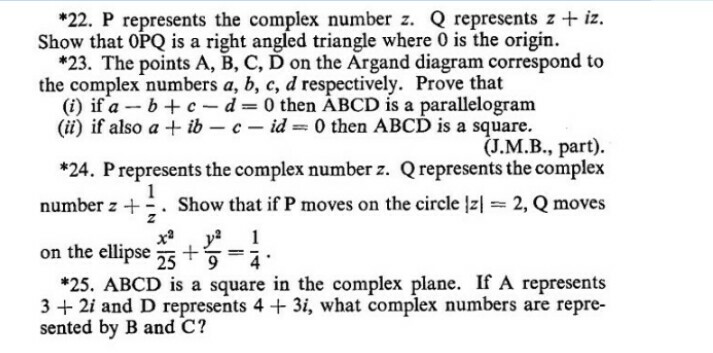
Question Number 60219 Answers: 1 Comments: 1
Question Number 60241 Answers: 0 Comments: 1

Question Number 60240 Answers: 0 Comments: 1
Question Number 60238 Answers: 0 Comments: 0

Question Number 60212 Answers: 1 Comments: 1

Question Number 60203 Answers: 2 Comments: 1
Pg 1510 Pg 1511 Pg 1512 Pg 1513 Pg 1514 Pg 1515 Pg 1516 Pg 1517 Pg 1518 Pg 1519
