Question Number 178018 by Spillover last updated on 12/Oct/22
![8.1g of a compound Q contain magnessium and oxygen.if mass of magnessium is 4.9g Determine the empirical formular of compound Q. [given Mg=24 O=16]](Q178018.png)
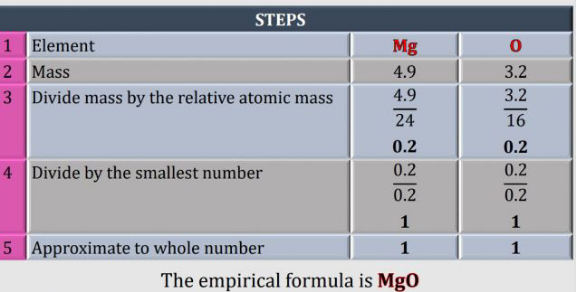
$$\mathrm{8}.\mathrm{1g}\:\mathrm{of}\:\mathrm{a}\:\mathrm{compound}\:\mathrm{Q}\:\mathrm{contain} \\ $$$$\mathrm{magnessium}\:\mathrm{and}\:\mathrm{oxygen}.\mathrm{if} \\ $$$$\mathrm{mass}\:\mathrm{of}\:\mathrm{magnessium}\:\mathrm{is}\:\mathrm{4}.\mathrm{9g} \\ $$$$\mathrm{Determine}\:\mathrm{the}\:\mathrm{empirical}\:\mathrm{formular} \\ $$$$\mathrm{of}\:\mathrm{compound}\:\mathrm{Q}. \\ $$$$\left[\mathrm{given}\:\mathrm{Mg}=\mathrm{24}\:\:\:\mathrm{O}=\mathrm{16}\right] \\ $$
Answered by Spillover last updated on 12/Oct/22
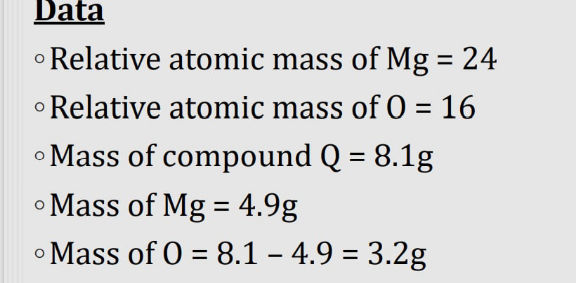
Answered by Spillover last updated on 12/Oct/22